1162
Views & Citations162
Likes & Shares
Radiation
therapy is the standard of care for a variety of oncological conditions because
it is highly effective at reducing the risk of local recurrence following
resection. However, radiation often causes detrimental effects to the
surrounding tissue. One therapeutic outlet being increasingly explored to
address post-radiation morbidities is autologous fat grafting (AFG). Beneficial
regenerative effects including volume restoration, improved skin quality, pain
reduction, and trophic restoration are attributed mainly to the presence of
adipose-derived stem and stromal cells present within the adipose tissue. Here
we review published data pertaining to the use of autologous fat grafting and
cell-assisted lipotransfer for the treatment of radiotherapy-associated tissue
damage, or radiodermatitis, as well as the data supporting the role of
adipose-derived stem cells.
INTRODUCTION
Radiation therapy is the standard of care for a variety of oncological
conditions because it is highly effective at reducing the risk of local
recurrence following resection. However, radiation often causes detrimental
effects to the surrounding tissue including skin discoloration altered collagen
structure, reduced skin elasticity, and dermal thickening as well as a
reduction in small vessel density, blistering, draining and/or dryness [1,2].
More serious cases can produce chronic pain, scarring, ulceration and tissue
necrosis. The structural alterations and complications of the tissue can make
reconstruction of irradiated wounds and tissue difficult.
One therapeutic outlet being increasingly explored to address
post-radiation morbidities is autologous fat grafting (AFG). Autologous fat
grafting (AFG) is steadily becoming the method of choice for most contour and
soft tissue defect repairs. AFG was first reported in 1893 by Gustav Neuber to
fill a depressed facial scar [3]. Poor clinical results and interference with
xenography and mammograms caused AFG to be overlooked for nearly a century
[4,5]. Recently, however, improvements in imaging techniques and clinical
outcomes have enabled AFG to become an increasingly popular remedy for a myriad
of reconstructive problems requiring soft tissue restoration. The use of
adipose tissue to treat soft tissue injuries and restore volume has
dramatically increased in the last 20 years. Adipose tissue makes an ideal
filler because it is easily acquired and prepared and ultimately provides a
permanent, natural filler. The clinical use of AFG to treat irradiated tissue
has demonstrated drastic regenerative effects in addition to restored volume
including improved skin quality, improved skin tone and improved structural and
vascular networks. These beneficial regenerative effects are attributed mainly
to the presence of adipose-derived stem and stromal cells present within the
adipose tissue [6,7].
The benefit of adipose-derived stem cells in fat
grafting has been frequently explored in the last 10 years, beginning with the 2006 report by Matsumoto et al. [8] which demonstrated superior
volumetric retention of fat grafting when supplemented with adipose-derived
stem cells. They termed this technique cell-assisted lipotransfer (CAL).
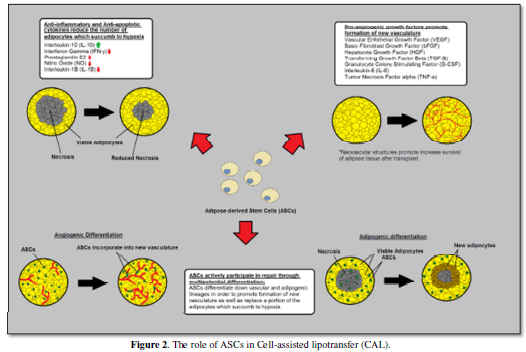
Adipose-derived stem cells are thought to facilitate graft survival by
producing anti-inflammatory, anti-apoptotic, and pro-angiogenic cytokines as
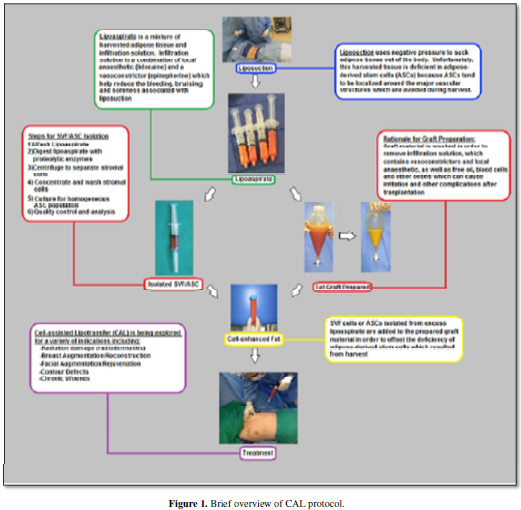
well as differentiating into adipose tissue and new vascular structures. Figure 1 and Figure 2 gives a brief overview of methodology of cell-assisted
lipotransfer and the role of ASCs. Here we review published data pertaining to
the use of autologous fat grafting and cell-assisted lipotransfer for the
treatment of radiotherapy-associated tissue damage, or radiodermatitis, as well
as the data supporting the role of adipose-derived stem cells.
Radiotherapy Tissue Damage
Radiodermatitis is an inflammatory condition characterized by erythema,
edema, epidermal thickening, pruritus (itchiness) and desquamation (skin
peeling). Radiodermatitis
is one of the most common side effects observed in patients who have
received radiation therapy for sarcoma, breast, anal, vulvar, and head and neck
cancers [9,10]. The acute stage of inflammation leads to a more chronic
fibrotic process (chronic radiodermatitis). Chronic radiodermatitis is
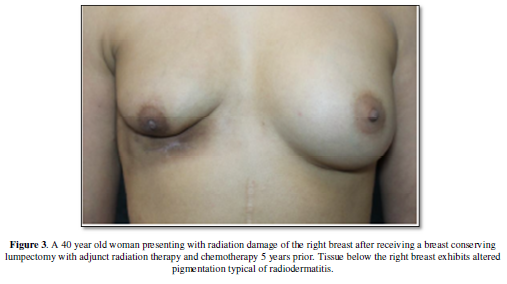
characterized by a decrease in elasticity, subpar wound healing, and
hyperpigmentation (Figure 3) and at
its most severe state, radionecrosis with ulceration [11-13].
Exposure to radiation has also been shown to significantly alter the
microvascular structure of exposed tissue. In the acute phase of the response
to radiation exposure, the vascular endothelium experiences increased
permeability, leading to edema and thrombosis. The hyperpermeability ultimately
leads to an increase in capillary density, but the newly formed capillaries are
irregular and easily occluded [14]. Damage to the vascular endothelium induces
hypoxia and exposure to ionizing radiation upregulates transforming growth
factor ß (TGF-ß) production by human dermal fibroblasts [15]. TGF-ß is a
cytokine which actively promotes radiation-induced fibrosis [16]. Radiation
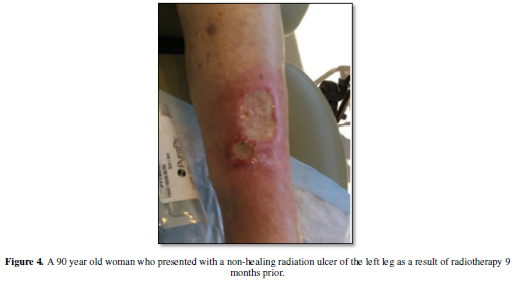
fibrosis predisposes the treatment area to the development of ulcers, skin
breakdown and tissue retraction, which can be painful and limit mobility (Figure 4). Additional morbidities which
can be observed in the chronic phase of radiodermatitis include loss of hair
follicles, nails, skin appendages and sebaceous glands in the treatment area.
Radiation induced tissue damage and ischemia eventually lead to tissue necrosis
if left untreated and the radiation exposure predisposes exposed cells to
neoplastic transformation, as ionizing radiation can cause mutations and
chromosomal abnormalities in mitochondrial and nuclear DNA [17]. Table 1 summarizes the characteristics
of acute and chronic radiodermatitis.
The level of tissue damage is commonly assessed using a variety of
assessment tools, none of which has emerged as a gold standard. The most widely
used grading scales are the National Cancer Institute's Common Terminology
Criteria for Adverse Events (CTCAE) for the classification of acute
radiodermatitis and the Radiation Therapy Oncology Group (RTOG)/European
Organization for Research and Treatment of Cancer (EORTC) scale or the Late
Effects Normal Tissue Task Force- Subjective, Objective, Management, and
Analytic (LENT-SOMA) scale for the classification of chronic dermatitis. Table 2 briefly summarizes all 3
grading scales for dermatologic conditions.
Autologous Fat Grafting
The first documented clinical series using autologous fat grafting to
treat radiotherapy tissue damage was published by Rigotti et al. in 2007 [18].
This study reported on the clinical outcomes of 20 patients treated with
autologous
lipoaspirate injections into irradiated breast tissue. All patients had
a LENT-SOMA grade 3 (severe) or grade 4 (irreversible functional damage)
radiation damage (Table 2) as a
result of receiving a dose of radiation between 45-55 Gy. Rigotti et al.
reported dramatic symptom improvement in 19 out of 20 subjects and overall a
significant decrease in the LENT-SOMA score after therapy. Symptomatic
improvements observed included complete remission of all cases of skin
necrosis, elimination of telangiectasia and pain, as well as a reduction of
fibrosis, atrophy and skin retraction. The 19 out of 20 patient who showed a
response to the therapy all experiences an improvement of 2 points or better on
the LENT-SOMA Scale.
Rigotti et al. examined the potential causes of these regenerative
effects at a cellular level. Prior to injecting any lipoaspirate back into
patients, compositional and ultrastructural analysis were conducted on the
harvested lipoaspirate. What they found attributed the regenerative effects of
the tissue to the stromal cell populations contained within the lipoaspirate,
specifically the adipose-derived stem cells. Ultrastructural analysis revealed
a relative paucity of fully intact adipocytes and an abundance of intact
stromal cells. Histologically they observed progressive regeneration including
neovascular formation and improved tissue hydration. They ultimately suggested
the presence of adipose-derived stem cells to be responsible for neovascular
formation and improved tissue quality.
Sultan et al. [19] examined the effects of autologous fat grafting on
chronic radiodermatitis in a murine model in an attempt to clarify the
mechanism of regeneration observed by Rigotti et al., with an emphasis on
histological and phenotypic changes. A group of 25 mice (wt FVB mice) were
divided into 3 groups: control (non irradiated, no injections, n=5), irradiated
and then sham grafted (saline injections, n=10) and irradiated and then fat
grafted (human fat injections, n=10). Sham and fat grafts occurred 4 weeks
after mice received a 45 Gy dose of radiation. They noted that in the fat
grafted group, radiation ulcers became smaller and hyperpigmentation decreased,
whereas no improvements
were noted in control or sham grafted groups. Histologically, they observed
that fat grafting attenuated epidermal thickening, stabilized irradiated
microvasculature, restored collagen organization and reduced the fibrotic
response to radiation.
A 2014 study by Garza et al. [18] further examined the effects of fat
grafting on irradiated tissue. A set of 15 Crl:NU-Foxn1nu
CD-1 immunocompromised mice were divided into 2 groups. 6 were used as
non-irradiated controls and 9 received a dose of 30 Gy of external beam
radiation to the scalp. 4 weeks post irradiation, all mice received 200ul
injections of donor human fat into the scalp. Skin samples were harvested
before fat injections (4 weeks after irradiation) as a baseline and at 2 and 8
weeks post fat injection. They observed that fat grafting alleviated dermal
thickening which resulted from radiation exposure, decreased skin collagen
content and increased neovascular density in irradiated skin. However, they
noted significantly decreased fat retention observed in the irradiated tissue
compared to the control group, but the permanently engrafted tissue was
histologically identical between groups. They attribute the decreased
volumetric retention to the greater ischemic state resulting from irradiation.
When the graft material, already deficient in vasculature as a result of harvest
[20], is introduced into the ischemic environment of the irradiated tissue, it
is exposed to a greater level of hypoxia than it would if placed into healthy
tissue, thereby decreasing the overall retention as a greater number of mature
adipocytes succumb to hypoxic stress.
Salgarello et al. [21] reported another clinical series involving the
treatment of 16 patients who received a prior mastectomy or lumpectomy with
adjunct radiation therapy. All patients had radiation damage at grade 1 or 2 on
the LENT-SOMA scale. All subjects were treated with autologous fat grafting to
the affected region and required 2 or 3 sessions in 3 month increments in order
to achieve a
LENT-SOMA score of 0 (no damage). Salgarello et al. reports high
patient satisfaction in all cases except 1, and additionally that the use of
fat grafting can help reduce the risk of implant related complications in
patients with a history of radiation if treated with AFG prior to placement of
an implant.
Phulpin et al. [22] reported on a series of 11 patients who received
AFG to alleviate radiotherapy induced tissue damage in the head and neck area.
10 patients had received 50 Gy of radiation or more and 1 patient received a
dose of 30 Gy. AFG was conducted throughout affected areas. Improvements
reported included disappearance or decrease in fibrosis, restoration of tissue
volume and symmetry as well as other significant aesthetic improvements. In
addition to cosmetic improvements, restoration of function was reported in a
number of cases as well including easier swallowing, increased mobility of the
head and neck region, improvement of breathing, and improved phonation.
A number of case reports describing treatment of 1 or 2 patients for
radiotherapy tissue damage have been published as well [23-25]. These studies
all report favorable outcomes in terms of tissue quality, aesthetics and
patient satisfaction where applicable. An additional benefit which reported for
AFG is the reduction of pain in patients experiencing post-mastectomy pain
syndrome (PMPS) after undergoing mastectomy with radiation therapy [26,27].
Cell-assisted Lipotransfer
The observations of Garza et al. suggest that further research into
methods focused on improving the volumetric retention of autologous fat
grafting in irradiated tissue is warranted. A study published in 2016 by Luan
et al. [28] examined the outcomes of AFG and CAL in a mouse model. A total of
24 Crl:NU-Foxn1nu CD-1 immunocompromised mice were divided
into 4 groups: irradiated with AFG (n=6), irradiated with CAL (n=6),
non-irradiated with AFG (n=6) and non-irradiated with CAL (n=6). Irradiated
mice received a dose of 30 Gy of external beam radiation. 5 weeks after
irradiation, all mice received a 200ul fat grafts of donor human fat to the
scalp which were supplemented with 10,000 uncultured SVF cells per graft
(50,000 SVF cells/mL) if in one of the CAL groups. There was no significant
difference in volumetric retention between mice who received CAL with or
without irradiation. Both CAL groups showed significantly greater volumetric retention
compared to the AFG groups. Following previously reported trends, the
irradiated AFG group resulted in significantly reduced volumetric retention
compared to all other groups. When comparing CAL and AFG in irradiated tissue,
CAL was shown to significantly increase graft integrity and decrease the
occurrence of oil cysts and vacuoles. CAL also resulted in a greater reduction
in dermal thickness, greater reduction in collagen density, and greater
increase in graft vascularity than AFG post engraftment. Overall, they noted
that CAL improved volumetric retention and provided greater rescue from
radiation-induced skin damage than AFG.
The Regenerative Potential of Adipose-derived
Stem Cells
As is suggested by the work of Rigotti et al. [6], Sultan et al. [7],
and Garza et al. [18], the regenerative effects of fat grafting are attributed
to the adipose-derived stem cell (ASC) population present within adipose
tissue. ASCs have been shown to play a supportive role in adipogenesis and
angiogenesis as well as a protective role by modulating inflammation and
immunity through cytokine production [20,29-33]. Numerous preclinical and
clinical studies has demonstrated a wide range of beneficial regenerative
effects exerted by adipose-derived stem cells.
Multilineage differentiation potential, specifically adipogenic and
angiogenic, is a very important aspect to the regenerative potential of ASCs
and is particularly important in terms of graft retention and volume retention.
Fat injections have been shown to result in growth of new adipose tissue at the
site of injection and paracrine stimulation by injected ASCs has been shown to
influence the local stem cell populations to differentiate down adipocyte
lineages [34,35]. When conducting fat transfer procedures, a significant
portion of the transplanted tissue will succumb to the hypoxic stress and die.
The presence of ASCs in the fat allows a portion of this tissue to regenerate
new adipose tissue as a result of adipogenic differentiation. Eto et al.
proposed a model for the fate of adipose tissue after transplantation [20]. The
system describes 3 tissue zones of transplanted fat: the surviving zone, the
regenerating zone and the necrotizing zone. The surviving zone receives
adequate oxygen via diffusion from the surrounding tissue and allows both the
fat and stem cell population to survive. The necrotizing zone is too deep and
insufficient oxygen reaches the tissue resulting in death and resorption of
both the adipocytes and stem cells. The regenerating zone however receives an
intermediate amount of oxygen which results in death of the adipocyte
population but survival of the stem cell populations, which are more resistant
to hypoxic insult. The surviving stem cell populations in the regenerating zone
tend to differentiate down adipocytic lineages and replace a portion of the
adipose tissue which was lost [20,30,31].
The angiogenic potential of ASCs is well documented as well. ASCs have
been shown to increase tissue perfusion in grafted areas via induction of
angiogenesis, through differentiation and paracrine mechanisms, as well as
playing a protective role on existing vasculature [29,32,36].
The presence of ASCs in grafted fat tissue promotes a more rapid
recovery from the hypoxic state experienced after transplantation. A more rapid
recovery of tissue perfusion and vasculature results in a greater survival rate
of grafted tissue as well as facilitate more rapid wound healing [37,38].
While it was initially assumed that the regenerative benefits of stem
cells was purely due to direct differentiation and replacement of damaged
tissues by the transplanted stem cell populations, growing evidence shows that
the greatest benefit is afforded by the paracrine effects of molecules secreted
by mesenchymal stem cells. The paracrine effects exerted by ASCs have
demonstrated anti-inflammatory, antioxidant, antiapoptotic and immunomodulatory
effects which protect neighboring cells against hypoxia, ischemia reperfusion
and reactive-oxygen species (ROS) induced damage as well as promote granular
tissue formation, reduce fibrosis, promote extracellular matrix remodeling and
increase epithelialization [39-41]. The secretory profile of adipose-derived
stem cells has been shown to secrete a wide variety of cytokines including
IL-8, IGF, bFGF, HGF and VEGF. These cytokines have all been associated with
vascular regeneration [42-44]. Given that a significant underlying cause of
radiotherapy induced tissue damage is associated with hypoxia, poor vascularity
and lymphedema, this secretion profile proves beneficial in the regeneration of
the microvascular environment. These immunomodulatory and proangiogenic
secretion profiles are shown to be strengthened in a hypoxic environment, like
that experienced in irradiated tissue [45,46].
CONCLUSIONS
There is a growing body of evidence in favor of the use of autologous
fat grafting or cell-assisted lipotransfer for the treatment of radiotherapy
tissue damage. In vitro analysis points to the presence of adipose-derived stem
cells contained within the transplanted tissue as being primarily responsible
for the regeneration. ASCs have demonstrated multi differentiation capacity as
well as a secretome which is proangiogenic, antiapoptotic and immunomodulatory,
all of which prove beneficial when overcoming injuries resulting from
radiotherapy. The data suggests that CAL provides a superior method of treating
radiation induced injuries compared to AFG, but a lack of well controlled
clinical trials and a relative paucity of clinical data has restricted more
widespread clinical adoption.
- Ryan
JL (2012) Ionizing radiation: the good, the bad, and the ugly. J Invest
Dermatol 132: 985-993.
- Hymes
SR, Strom EA, Fife C (2006) Radiation dermatitis: clinical presentation,
pathophysiology, and treatment 2006. J Am Acad Dermatol 54: 28-46.
- Neuber
GA (1893) Verhandlungen der Deutschen Gesellschaftfür Chirurgie 1: 66
- Delay
E, Garson S, Tousson G, Sinna R (2009) Fat injection to the breast:
technique, results, and indications based on 880 procedures over 10 years.
Aesthetic Surg J 29:
360-376 .
- ASPRS
(1987) Ad-Hoc Committee on New Procedures. Plast Surg Nurs 7: 140-141.
- Rigotti
G, Marchi A, Galie M, et al. (2007) Clinical Treatment of radiotherapy
tissue damage by lipoaspirate transplant: A healing process mediated by
adipose-derived adult stem cells. Plast
Reconstr Surg 119: 1409.
- Sultan
SM, Stern CS, Allen Jr. RJ, et al. (2011) Human fat grafting alleviates
radiation skin damage in a murine model. Plast Reconstr Surg 128: 363.
- Matsumoto
D, Sato K,Gonda K, et al. (2006) Cell-Assisted Lipotransfer: Supportive
Use of Human Adipose-Derived Cells for Soft Tissue Augmentation with
Lipoinjection. Tissue Engineering 12: 3375-382
- Salvo
N, Barnes E, van Draanen J, Stacey E, Mitera G, et al. (2010) Prophylaxis
and management of acute radiation-induced skin reactions: a systematic
review of the literature. Curr Oncol 17: 94-112.
- McQuestion
M (2011) Evidence-based skin care management in radiation therapy:
clinical update. Semin Oncol Nurs 27: e1-17
- Goldschmidt
H, Sherwin WK (1980) Reactions to ionizing radiation. J Am Acad Dermatol
3: 551-579.
- Bentzen
SM, Thames HD, Overgaard M (1989) Latent-time estimation for late
cutaneous and subcutaneous reactions in a single-follow-up clinical study.
Radiother Oncol 15: 267-274.
- Arcangeli
G, Friedman M, Paoluzi R (1974) A quantitative study of late radiation
effect on normal skin and subcutaneous tissues in human beings. Br J
Radiol 46: 44-50
- Morgan
K (2014) Radiotherapy-induced skin reactions: prevention and cure. Br J
Nurs 23: S26-32.
- Mu¨ller
K, Meineke V (2011) Radiation-induced mast cell mediators differentially
modulate chemokine release from dermal fibroblasts. J Dermatol Sci 61:
199-205
- Amber
KT, Shiman MI, Badiavas EV (2014) The use of antioxidants in
radiotherapy-induced skin toxicity. Integr Cancer Ther 13: 38-45
- Pie´rard
GE, Pie´rard-Franchimont C, Paquet P, Quatresooz P (2009) Emerging
therapies for ionizing radiation-associated skin field carcinogenesis.
Expert Opin Pharmacother 10: 813-821.
- Garza
RM, Paik KJ, Chung MT, et al. (2014) Studies in fat grafting: Part III.
Fat grafting irradiated tissue: Improved skin quality and decreased fat
graft retention. Plast Reconstr
Surg 134: 249-257.
- Sultan SM, Stern CS, Allen Jr. RJ, et al. (2011) Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg 128: 363.
- Eto H, Kato H, Suga H, et al. (2012) The fat of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg 129: 1081-1092.
- Salgarello M, Visconti G, Barone-Adesi L (2012) Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg 129: 317-329.
- Phulphin B, Gangloff P, Tran N, et al. (2009) Rehabilitation of irradiated head and neck tissues by autologous fat transplaantation. Plast Reconstr Surg 123: 1187.
- Ibchigolo F, Tatullo M, Pacifici A, et al. (2012) Use of dermal-fat grafts in the post-oncological reconstructive surgery of atrophies in the zygomatic region: clinical evaluations in the patients undergone to previous radiation therapy. Head Face Med 8: 33.
- Hespe GE, Albornoz CR, MEhrara BJ, et al. (2013) CASE REPORT Pharyngocutaneous fistula closure using autologous fat grafting. Eplasty 9: 13.
- Salgarello M, Visconti G, Farallo E (2010) Autologous fat grafting in radiated tissue prior to alloplastic reconstruction of the breast: report of two cases. Aesthet Plast Surg 34: 5-10.
- Caviggioli F, Maione L, Klinger F, et al. (2016) Autologous fat grafting reduces pain in irradiated breast: a review of our experience. Stem Cells Int.
- Maione L, Vinci V, Caviggioli F (2014) Autologous fat graft in postmastectomy pain syndrome following breast conservation surgery and radiotherapy. Aesthet Plast Surg 38: 528-532.
- Luan A, Duscher D, Whittam J, et al. (2016) Cell-assisted lipotransfer improved volume retention in irradiated recipient site and rescues radiation-induced skin changes. Stem Cells 34: 668-673.
- Rehmam J, Traktuev D, Li J, et al. (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109: 1292-1298.
- Suga H, Eto H, Aoi N, et al. (2010) Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 126:1911-1923.
- Kato H, Mineda K, Eto H, et al. (2014) Degeneration, regeneration and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 133: 303-313.
- Planat-Bernard V, Silvestre JS, Cousin B, et al. (2004) Plasticity of human adipose lineage cells towards endothelial cells: physiological and therapeutic perspectives. Circulation 109: 656-663.
- Miranville A, Heeschen C, Sengenes C, et al. (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349-355.
- Naderi N, Wilde C, Haque T, et al. (2014) Adipogenic differentiation of adipose-derived stem cells in a 3-dimensional spheroid culture (microtissue): implications for the reconstructive surgeon. J Plast Reconstr Aesthet. Surg.
- Zuk PA, Zhu M, Mizuno H, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-229.
- Baer PC and Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 3: 1–11
- Ebrahimian TG,Pouzoulet F,Squiban C, et al. (2009) Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 29: 503-510.
- Yuan Y, Gao J, Liu L, et al. (2013) Role of adipose-derived stem cells in enhancing angiogenesis early after aspirated fat transplantation: induction or differentiation? Cell Biol Int 37: 547-550.
- Kim WS, Park BS, Kim HK, et al. (2008) Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 49: 133-142.
- Mohammadzadeh A, Pourfathollah AA, Shahrokhi S, et al. (2014) Immunomodulatory effects of adipose-derived mesenchymal stem cells on the gene expression of major transcription factors of T cell subsets. Int Immunopharmacol 20: 316-321.
- Maria AT, Toupet K, Maumus M, et al. (2016) Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun.
- Huo Y, Ryu CH, Jun JA, et al. (2014) IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int 38: 1050-1059.
- Haleagrahara N, Chakravarthi S, Mathews L (2011) Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulated neovascularization in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci 12: 8562-8574.
- Sezer O, Jakob C, Eucker J, et al. (2001) Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur J Haematol 66: 83-88.
- Frazier TP, Gimble JM, Kheterpal I, et al. (2013) Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 95: 2286-2296.
- An HY, Shin HS, Choi JS, et al. (2015) Adipose mesenchymal stem cell secretome modulated in hypoxia for remodeling of radiation-induced salivary gland damage. PloS One 10: e0141862.
- U.S. Department of Health and Human Services (2010) Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
- Radiation Therapy Oncology Group (2016) RTOG/EORTC Late Radiation Morbidity Scoring Schema. 2016.
- Khanna NR, Kumar DP, Laskar SG, et al. (2013) Radiation dermatitis: an overview. Indian J Burns 21: 24-31.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Alcoholism Clinical Research
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Spine Diseases